Xu Dong 1, Chen Hongshu 2, Wang Jieliang 2 Abstract: This paper introduces the principle of color change of photochromic materials, thermochromic materials and electrochromic materials, research status, conditions for restricting development and their applications in military camouflage, automobile, construction, textile and garment and daily necessities. Focus on electrochromic materials (conductive polymers, composite films made of inorganic and organic materials, other types of polymer electrochromic materials, etc.). On this basis, the development trend of color-changing materials is forecasted, and it is pointed out that electrochromic materials are the future development trend. Slip-on Flange,Weld Neck Flange,Pipe Flanges Province Gold Mysterious Pipe Co., Ltd. , http://www.hbseamlesspipe.com
(1.School of Materials and Chemical Engineering, Xi'an Technological University, Xi'an 710032, China; 2.Institute of General Construction Engineering, Xi'an 710032, China)
Key words: photochromism; thermochromism; electrochromism; conductive polymer CLC number: TB332 Document code: AArticle ID:1004-244X(2011)03-0087-05
Discoloration refers to a change in the response to light produced by a substance under the influence of the external environment. This phenomenon is ubiquitous in nature. What is of interest to it is a reversible discoloration phenomenon, and devices made using the discoloration of materials are called color-changing materials. Under the action of external excitation sources (light, heat, electricity, etc.), the phenomenon that a substance or a system changes color significantly is called discoloration. Discoloration can be observed in gases, liquids or solids. According to the type of material, it can be classified into an inorganic color changing material and an organic color changing material. According to the stimulation mode of the material, there are mainly four categories: photochromism, thermochromism, electrochromism and other discoloration. Each of them has its application value.
The chemical mechanisms of color development of color-changing materials are: 1) atomic excitation and molecular vibration; 2) changes in the energy levels of transition metal atoms in the coordination field; 3) conjugation effects and organic dyes; 4) charge transfer effects and pigments, 5) Electrons transition between solid energy bands to produce color changes.
From the analysis of the current status and research dynamics of visible light stealth technology [1], it can be seen that electrochromic materials are superior to thermochromic materials and photochromic materials due to their superior performance, and may become the first smart cover for dynamic stealth. This stealth technology overcomes the inherent flaws of camouflage and camouflage net stealth, improving the mobility of the target, the scope of operations, and the ability to fight around the clock. The author focuses on electrochromic materials.
1. Research Status of Organic Color-Change Materials 1.1 Photochromic Materials Photochromic is a chemical-physical phenomenon. When a compound A is irradiated with light of a certain wavelength, a specific chemical reaction can occur to obtain the product B. Significant changes in the absorption spectrum due to structural changes. It can be restored to its original form under the illumination of another wavelength of light or by heat [2-3]. The mechanism of discoloration of organic photochromic materials is the cleavage and combination of double bonds (isolation of bonds and homogenization of bonds), formation of isomers (proton transfer tautomerization and cis-trans isomerization), redox reaction, week Ring reaction. The most important characteristics of organic photochromic materials with practical application prospects are that the color bodies must have sufficient thermal stability and the fatigue resistance of the photochromic compounds. At present, most studies on organic photochromism focus on diarylethene, spiropyran, spirooxazine, fulgide, and azo, and continue to explore and discover new photochromic systems.
For aircraft, warships, tanks, armored vehicles, etc., the surface is photochromic by coating or doping a photochromic material. Discoloration under illumination, matching with the environment, to achieve the purpose of being covered. National Cach Register of the United States has done a lot of research on how to match the equipment, personnel and environment colors to achieve camouflage. It applies photochromic materials to various ordnance as camouflage [4]. At present, photochromic camouflage has become the main way of visual stealth.
Photochromic fiber refers to a fiber that can undergo a reversible change in color under the irradiation of sunlight or ultraviolet light. As early as the 1970 Vietnam War, photochromic compounds were applied to clothing by the US military to achieve military camouflage [5]. The yarns produced by Solar Active International of the United States are available in various colors such as orange, purple, blue, magenta, yellow, red and green under ultraviolet light. In recent years, Clemson University and Georgia Institute of Technology in the United States are exploring the incorporation of color-changing dyes into optical fibers and changing the surface coating materials of optical fibers to enable automatic control of fiber color. US military researchers believe that the combination of optical fibers and color-changing dyes can ultimately achieve automatic color changes in clothing.
1.2 Thermochromic materials Thermochromic materials are functional materials whose color changes significantly with temperature in a certain temperature range [6]. The thermochromism is due to the reversible change in the spectral properties of the color-changing material, strictly speaking, limited to changes in the visible range [7]. There are two kinds of thermochromic discoloration mechanisms, which are discolored due to physical changes (such as crystal transformation, lattice expansion and contraction, loss of crystal water and moisture absorption) after heating, such as: reversible color-changing materials; chemistry due to heat generation Change (decomposition, compounding) and discoloration, such as: irreversible color changing materials. As far as the research on reversible color-changing materials is concerned, many patented technologies have been published, especially in Japan [8-12]. At present, the development of thermochromic materials tends to be both low temperature and reversible [13], among which organic reversible thermochromic materials are attractive because of their sensitive color and rich color. Its application extends from simple temperature display to industrial, anti-counterfeiting and daily decoration.
1.3 electrochromic material electrochromic [14] (Electrochromic, EC) refers to the phenomenon that the optical properties of the material produce a stable and reversible color change under the action of an applied electric field, and the appearance performance is reflected in the reversible change of color and transparency. . The discoloration mechanism of organic electrochromism mainly depends on the chemical composition band structure and redox characteristics of the material. The doping and dedoping of ions and electrons modulate the absorption characteristics of the film in the visible region or change the carrier concentration in the film. And the plasma oscillation frequency to achieve modulation of the infrared reflection characteristics. These films should be transparent in the state of no discoloration, and the discoloration is reversible. When a current is passed, the electrochromic film produces a color, and the depth of the discoloration can be controlled by the magnitude of the current passed, and the original color remains unchanged after the current is cut off. To fade it, just add a reverse current. Therefore, it has broad application prospects in display devices, automobiles, military camouflage [15], smart materials [16], energy-saving building materials and other fields.
According to "New Scientist" magazine, the chemist of the University of Connecticut, Goreg Sotchenko, invented a wire spun from an electrochromic polymer. The clothes made of this wire can be used under the electric field. Feel free to change colors. When the voltage changes, the electron energy in the wire also changes, which causes the wavelength of the light absorbed by the electron to be different, so the color of the clothes changes. At present, Goreg Sotger has been able to perfectly change the color of this silk from orange to sky blue and from red to sky blue.
The research group led by Prof. Peng Huisheng from Fudan University in China [17] first developed a composite fiber with environmentally sensitive polymer material polydiyne and carbon nanotubes, and developed a new intelligent material with electrochromic energy. Change or restore colors quickly. At present, there are mainly three types of electrochromic materials to be studied.
1.3.1 Composite films made of inorganic and organic materials Since the discovery of the electrochromic effect of transition metal oxides in 1960, such materials have become a research hotspot for more than 40 years [18]. The electrochromic properties of transition metal oxides such as WO3, MoO3, TiO2, IrO, NiO, etc. have been discovered. The most research is the discoloration of WO3 film. It is found that WO3 has high coloring efficiency, good reversibility, short response time and long life. The low cost and other advantages are considered to be one of the most promising electrochromic materials. As a color-changing material, nickel oxide film has a large coloring, achromatic wavelength range, good cycle color change life and a rich source of raw materials, making it the most promising material for large-area electrochromic devices after WO3 film. The methods for preparing the film mainly include electron beam evaporation, pulsed laser deposition, direct current sputtering, electrochemical deposition, sol-gel method, etc. [19-25], as shown in Table 1. It can be seen that the sol-gel method has the advantages of simple process, low equipment cost, change of electrochromic effect by doping and easy preparation of large-area film, and other methods are limited due to expensive equipment and complicated technology. 
Yao et al [26] prepared the WO3 electrochromic film by sol-gel method. By optimizing the process parameters, it was found that the film was continuously coated on ITO at a pulling speed of 5 cm/min and heat treated at 250 ° C for 60 min. The film has good electrochromic properties. The doping amount [27] also has an effect on the response time and applied voltage of the WO3 film. Domestic research institutes such as the Shanghai Institute of Ceramics of the Chinese Academy of Sciences [28] and the School of Aerospace and Materials Engineering of the National University of Defense Technology [29] have studied the MoO3 electrochromic film through the doping of Li+ and the flexible matrix (PET). The choice of MoO3 electrochromic film.
1.3.2 Main chain conjugated conductive polymer conductive polymer has the advantages of low density, diverse structure, unique physical and chemical properties, generally 1~2 g/cm3, and conductivity can be widely doped. Adjustment within range. The disadvantage is that it is difficult to process due to insolubility and infusability [30]. Except for polyaniline, there is no commercial production, and the price is very expensive. Conductive polymers have very good development prospects [31]. The main development directions include: the use of controllability of conductivity, color and other characteristics, the development of intelligent stealth materials; the use of easy processing, fiber-forming properties, mixed with carbon fiber, etc., the development of multi-functional color-changing fibers; to "thin, light, The development of wide-type color-changing materials and film materials.
Compared with other conductive polymer materials, polypyrrole and polyaniline have broad application prospects because of their good environmental stability, high electrical conductivity, large variation range and easy synthesis. For example, since polypyrrole and polyaniline synthesized under normal conditions are insoluble and infusible and difficult to process, a method of substituent modification and doping is usually used to improve processability, but the enhanced processing performance is usually at the sacrificial conductivity. The price. The ideal method is to form a specific morphology directly in the process of polymerization. Nanotubes and nanowires are directly formed nanomaterials with linear morphology, and the unique properties of nanomaterials make them both electrically Magnetic double-function microwave absorption characteristics [32], thus achieving the optical, electrical and magnetic multi-functional properties of nanotubes and nanowires. Researchers at home and abroad have used different methods to prepare conductive polymer nanotubes and nanowires, which has become a hot research topic in recent years.
Zeng Xianwei et al. [33] used vertical furnace flow method, using ferrocene as catalyst, thiophene as cocatalyst, benzene as carbon source to prepare carbon nanotubes, and uniformly coated polyaniline on the surface of carbon nanotubes by in-situ polymerization. A carbon nanotube/polyaniline one-dimensional nanocomposite was prepared. Figure 1 is a scanning electron micrograph and a transmission electron micrograph of a carbon nanotube/polyaniline composite, respectively. 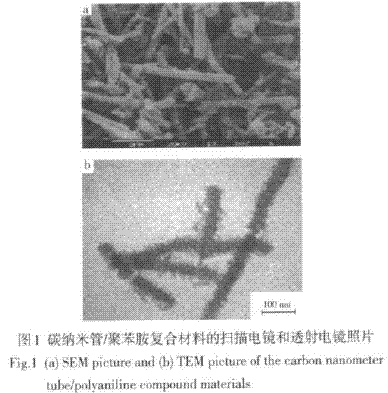
Electrochromic fabrics are the chameleon fabrics that people dream of. It can maintain its own color through weak current regulation and is consistent with the external environment. It has important application value in military camouflage. Therefore, the military and scientists all over the world have invested huge amounts of money and energy in order to make practical breakthroughs in this respect.
Domestic universities such as Donghua University, State Key Laboratory of Fiber Materials Modification [34-35], Sichuan University [36], Beijing Key Laboratory of Research and Development and Evaluation of Clothing Materials [37], etc. are currently conducting composite conductive Fiber research. They modified the polyester fiber separately. Through experimental analysis and comparison, it is known that two times of mechanical extrusion and alkali solution treatment of polyester are introduced in the preparation process, and the composite conductive fiber prepared has excellent electrical conductivity and surface resistance can be reduced to 102. ~103Ω orders of magnitude. Li et al. [38] prepared polyaniline-based conductive cotton cloth (PANI/CCT) by "in situ" polymerization of aniline on white cotton substrate with ammonium persulfate as oxidant, as shown in Fig. 2. It can be seen from Figures 2a and b that the surface of the pure cotton cloth is smooth. And in Figures 2c~f, it can be seen that polyaniline is densely and uniformly coated on the surface of a single fiber. 
It is found that the prepared PANI/CCT has electrochromic properties, yellow-green in -0.45~0.3 V, and dark green in 0.3~1.0 V, which is expected to be applied in the preparation of all-solid electrochromic fabrics. .
1.3.3 Other types of polymer electrochromic materials 1.3.3.1 Metal phthalocyanine Since 1970 Lu (Pc) 2 electrochromic film material has been put into use after vacuum evaporation, a series of 酞花é’电A color-changing material with a metal ion in the center. Domestic Zhang Xin et al [39] synthesized tetranaphthyl cadmium phthalocyanine compounds by melting method using 4-nitrophthalic acid, cadmium chloride, urea and ammonium molybdate as raw materials.
Zhang Jidong et al. [40] studied the near-infrared electrochromism of indigo indigo, using a vacuum deposited film method to obtain a green film in a neutral state, in a 1.0 V oxidation state (orange) - 0 V. Behavior (green)—The color change between the 0.1 V reduction states (blue). This method realizes the preparation of the film, has high cost and complicated operation.
1.3.3.2 Metal-organic complexes Ye Kaiqi et al [41] synthesized a novel boron complex blue electroluminescent material (phenolic pyridine fluoride boron fluoride PPBF2, PP: 2-(2-phenolic pyridine)). The complex PPBF2 showed strong blue fluorescence (440 nm) in both solution and solid state. By using it as an electroluminescent material, depending on different device structures, different colors of light can be observed, and it is desired to achieve more efficient electroluminescence by adjusting the material structure and improving the device configuration.
1.4 Other color-changing materials In addition to the above three color-changing materials, there are also pressure-sensitive color-changing materials, solvent-chromic materials, and the like. The pressure-sensitive color-changing material can change its color when subjected to pressure or pressure changes, which is caused by the phase change of the piezochromic material [16]. For example, after applying a layer of pressure-sensitive color coating on the outside of the load-bearing sling, when the load of the sling reaches or exceeds the warning quality, the color change of the sling warns the user to avoid a dangerous accident.
A solvent-chromic material is a phenomenon in which a color change occurs when a substance comes into contact with a specific solvent, which is called solvent-induced discoloration. Many substances produce different colors under different solvents. Organic species are mainly heteropolycyclic compounds due to their wide variety. The discoloration mechanism of the organic lyotropic material is solvent polarization or the like.
2. Restricting the development conditions of organic color-changing materials With the rapid development of modern science and technology, composite materials have been greatly improved in terms of preparation technology and performance and application, and are rapidly moving toward the direction of compounding and intelligentization. development of. At present, countries around the world are paying more and more attention to the research on color-changing materials. Many domestic units are committed to this research and have achieved stage results. It is expected to make further breakthroughs in intelligent color-changing materials. However, most of the current color-changing materials such as photochromic materials, thermochromic materials, and electrochromic materials have disadvantages such as single color change and low cycle number. Many methods can only be completed in the laboratory, and large-area use is limited. It is difficult to achieve the full color change of red orange yellow green blue blue purple and the requirement of large area use. When preparing color-changing materials, in addition to meeting the requirements of color, the material performance requirements are generally strict, such as conductivity, number of cycles, response time, and easy realization of large area.
3. Conclusions At present, foreign countries have made some developments in the research of new materials and technologies. Although the country started late in the research of color-changing technology, it has also achieved certain research results. In the study of color-changing materials, inorganic color-changing materials are much more mature than organic color-changing materials, but inorganic color-changing materials have the disadvantage of single color change and difficult design. For conductive polymers of organic color-changing materials, they have low cost and easy molecular design. , rich in color and easy to process. With the development of modern military, electrochromic materials are superior to photochromic materials and thermochromic materials in some respects. Therefore, research on new intelligent materials (electrochromic materials) is an inevitable trend and has become the direction of military camouflage research in the future.
4. References: slightly
Research progress on color changing materials