In recent years, a new type of two-dimensional material of graphene, silicon, has emerged in the field of silicon-based research. Silylene is also a Dirac fermion system, its low energy quasiparticles have a linear band structure, and it is also a two-dimensional topological insulator. In silylene, since the large bonding distance between Si-Si atoms weakens the π-electron overlap, it forms a low-buckled monoatomic layer honeycomb in the form of sp2-sp3 hybrid hybridization. The structure differs from the planar honeycomb structure formed by sp2 hybridization between CC atoms on graphene. This weak warping structure of siliconene produces many excellent electronic properties that are different from graphene, such as having a larger spin-orbit coupling energy gap, a stronger response to an electric field, and easier occurrence with foreign atoms and molecules. Interaction and so on. Since 2012, Institute of Physics, Chinese Academy of Sciences/Beijing National Laboratory for Condensed Matter Physics (National Laboratory for Condensed Matter Physics), Wu Kehui, a researcher at the State Key Laboratory of Surface Physics, and SF09, an associate researcher, have conducted systematic research on siliconene, not only leading the experiment. Siliconene was prepared [Nano Lett. 12, 3507 (2012); PRL 110, 085504 (2013)] and also revealed that the siliconene has a Dirac type electronic state [PRL 109, 056804 (2012); ACS Nano 7, 9049 ( (2013)] and a series of other features that have had a significant impact on the world of silicon research. Hydrogenation is an effective means of regulating the structure and electronic properties of two-dimensional materials. It is well-known that graphene can be obtained after hydrogenation of graphene, and solving the zero energy gap of graphene is not beneficial to the application of field effect transistors. Then, it is natural to assume that after the hydrogenation of silylene, plane silanes can also be obtained. Theoretical calculations have been reported on the adsorption configuration of hydrogen atoms on the surface of free-standing silylenes and their regulation on the electronic properties of silylenes. Related studies have found that the hydrogenation of silylene opens up larger bands. Gap, and semihydrogenated silylene also exists ferromagnetic. However, no hydrogenation has been reported experimentally for monolayers of siliconene prepared on Ag(111) substrates. Recently, Wu Kehui and Chen Hao (SF09) collaborated with Meng Shenghe and associate researcher Li Hui, a researcher in the surface chamber SF10 group, using low temperature scanning tunneling microscopy/scanning tunnel microscopy (STM/STS) and density functional theory (DFT) calculations. Hydrogenation of a monolayer of siliconene on an Ag(111) substrate was studied. The adsorption process and adsorption structure of hydrogen atoms on the siliconene were studied for the first time. The adsorption mechanism of hydrogen atoms was clarified and the theory was obtained. On the semi-silane. The hydrogenation result also clarifies the structural problem of the (2√3×2√3)R30° phase, demonstrating that it is a monolayer of siliconene. Among the siliconenes grown on Ag(111) substrates, the 3×3 (relative to Si-1×1 lattice) structure of siliconene has the simplest structure and is the most clearly studied, and is currently recognized as a monolayer of siliconene. film. This 3×3 phase contains a stable phase (α-3×3) structure and a metastable phase (β-3×3) structure. The energy difference between the two is small and can coexist on the same surface. . Experimentally, after saturated hydrogenation of the 3×3 phase, an ordered structure with the same 3×3 cycle can be obtained, which is called γ-3×3 structure. This is in contrast to the disordered cluster structure obtained after hydrogenation of graphene. In sharp contrast. Combined STM and DFT calculations found that the adsorption of hydrogen atoms will change the warping configuration of silicon atoms, resulting in hydrogenation of silylene from the α-3 × 3 phase to β-3 × 3 phase structural transformation. The mechanism of adsorption of hydrogen atoms on the silica can be explained qualitatively by the “sublattice adsorption†image, ie hydrogen atoms tend to adsorb on the same daughter lattice of the siliconene. In addition, the hydrogen atoms adsorbed on the silylene have a lower desorption temperature (about 450K), indicating that hydrogenation is a reversible process. These results indicate that silylene may be a hydrogen storage material. For the first time, the ordered hydrogenation structure of siliconene was obtained, and the adsorption mechanism of hydrogen atoms on the monolayer siliconene was clarified, which provided an experimental basis for the electronic regulation of silylene in the future. Related results have been published in Phys. Rev. Lett. 114, 126101 (2015). Among the various single-layered silylene phases grown on Ag(111) substrates, only the (2√3×2√3)R30° phase (relative to the Ag-1×1 lattice) can evenly cover the entire Ag ( 111) Surface. However, the structure of the (2√3×2√3)R30° phase is more complicated than that of the simple ordered 3×3 phase. The STM diagram shows disorder and defects, so some people suspect that it is a fragment of siliconene. Some people also suspect that it is a Si-Ag alloy. In a hydrogenation experiment, the researchers found that the (2√3×2√3)R30° phase can be clearly seen after the complete hydrogenation of the hidden silane-1×1 lattice, indicating that (2√3×2√) 3) R30° phase is actually a complete monolayer of siliconene. In addition, the (2√3×2√3)R30° phase hydrogenated structure is closer to the complete 1×1 lattice than the 3×3 phase, so it can be seen as a new two-dimensional material—semisilane (half-silicane) lays the foundation for subsequent studies on the electronic properties of silanes. The relevant results were published in the recent ACS Nano (DOI:10.1021/acsnano.5b04722). The graduate students who participated in the above-mentioned work mainly include Qiu Jingyi, Xu Yang (SF09 group) and Fu Huixia (SF10 group). This work was supported by funding from the National Natural Science Foundation of China (Project Number, 11322431, 11174344, 91121003), Chinese Academy of Sciences (project number: XDB07020100), and Ministry of Science and Technology (project number: 2012CB921703, 2013CBA01601, 2013CB921702).
The undermount sink is installed under the countertop, which has more space for use. The countertop is easy to clean and maintain. You can easily sweep the dirty water from the countertop into the sink with a rag, making it easier to clean the kitchen.Undermount sink Follow this video, you can easily install an undermount sink in just a few minutes. A must-have in the kitchen, a high-quality and easy-to-clean stainless steel sink can help you perform household chores efficiently. Undermount Sink,Undermount Kitchen Sink,Undermount Stainless Steel Kitchen Sink,Copper Undermount Sink JIANGMEN MEIAO KITCHEN AND BATH CO.,LTD , https://www.meiaogroups.com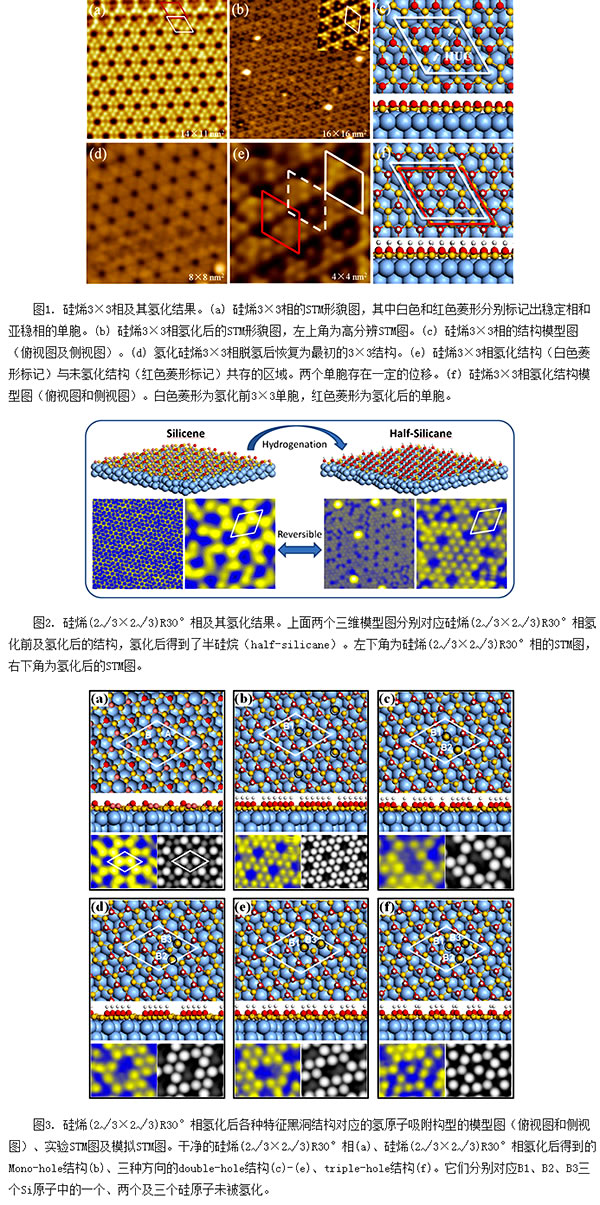
New progress in the hydrogenation of styrene by physics